Phase I Clinical Trial
Study Design:
The study involved participants with mild to moderate Alzheimer’s disease to evaluate the safety and tolerability of IGC-AD1.
Results: IGC-AD1 was found to be safe and tolerable at three different dosage levels.
– Improvements were observed in Neuropsychiatric Symptoms (NPS) and Caregiver Distress.
Objectives

Primary

Secondary

Demographics
Female
69.2%
Male
30.8%
Age
80.46 ± 5.71 y.o.
Weight
147.66 ± 31.62 lb.
Height
5.28 ± 0.46 ft.
BMI
21.58 ±
1.02
Phase I Clinical Trial Results
- IGC-AD1 is safe and tolerable at three different dosage levels.
- Caregiver distress, and Neuropsychiatric Symptoms (NPS), including agitation, anxiety, and depression improved.
Phase I Clinical Study Results
Results on NPS
Neuropsychiatric Symptoms Measured by NPI Scores
Patients taking IGC-AD1 intervention showed an overall improvement in NPS. Caregiver distress improved as well.
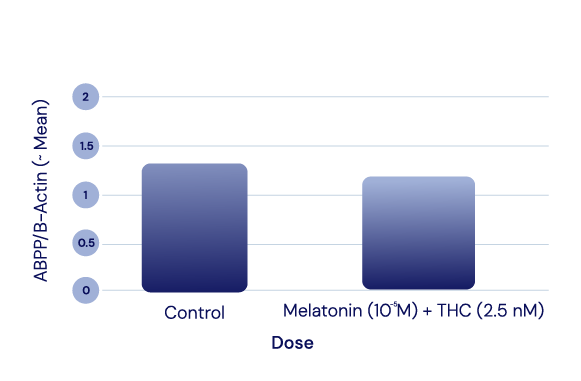
APP Levels
The APIs in IGC-AD1 did not reduce Amyloid Precursor Protein (APP) levels in Alzheimer’s cell lines. APP modulates cell growth, motility, and survival; it is cut to create small fragments such as the Aβ peptide that eventually deposit as plaque.