Pre-clinical
Preclinical studies have allowed us to identify the potential of IGC-AD1 to be an AD-modifying drug. The combination of the active ingredients at low, non-toxic concentrations was shown to reduce Aβ aggregation in N2aAβPPswe cells, maintain APP levels, and enhance mitochondrial function in a dose-dependent manner.
Pre-clinical
Study results
-
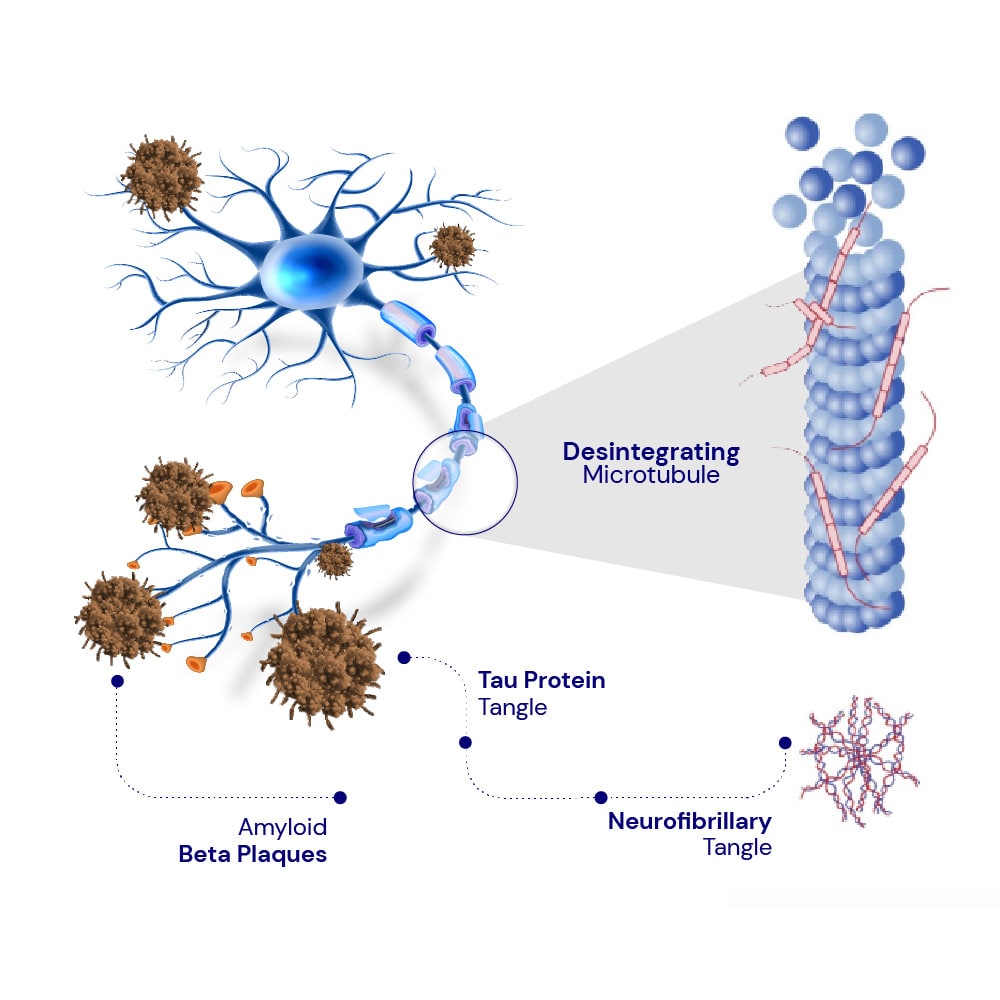
Inhibit the formation of neurofibrillary tangles
-
Inhibit the formation of plaques.
-
Enhance mitochondrial functioning.
-
Improve spatial memory.

Pre-clinical studies:
Detailed scientific results
The API in IGC-AD1 reduces Aβ40 peptide production and Aβ42 aggregation in Alzheimer’s cell lines.
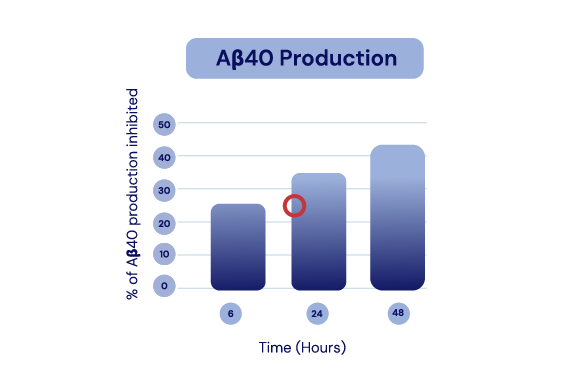
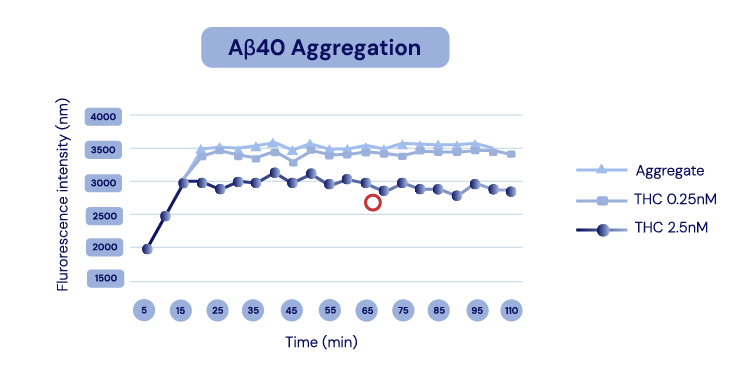
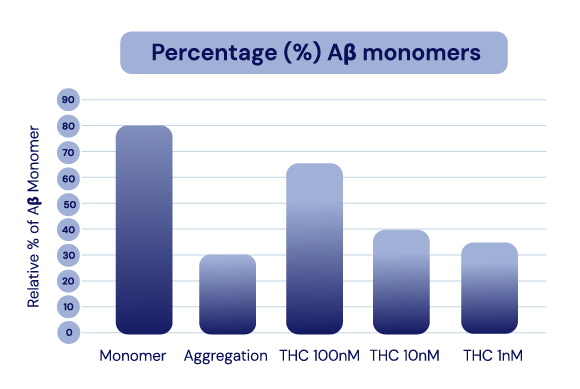
Representation of Cao et al., 2014
APP Levels
The APIs in IGC-AD1 did not reduce Amyloid Precursor Protein (APP) levels in Alzheimer’s cell lines. APP modulates cell growth, motility, and survival; it is cut to create small fragments such as the Aβ peptide that eventually deposit as plaque.
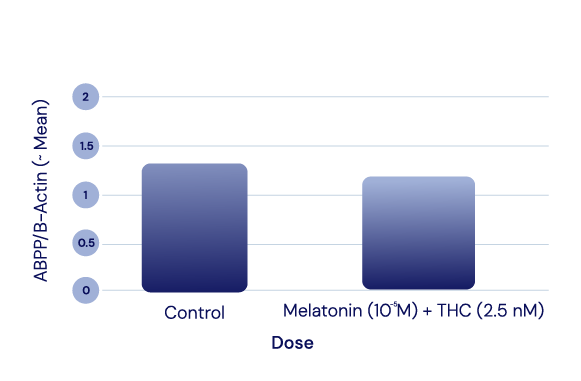
APP Levels
The APIs in IGC-AD1 did not reduce Amyloid Precursor Protein (APP) levels in Alzheimer’s cell lines. APP modulates cell growth, motility, and survival; it is cut to create small fragments such as the Aβ peptide that eventually deposit as plaque.

APP Levels
The APIs in IGC-AD1 did not reduce Amyloid Precursor Protein (APP) levels in Alzheimer’s cell lines. APP modulates cell growth, motility, and survival; it is cut to create small fragments such as the Aβ peptide that eventually deposit as plaque.

