Phase 2 interim results
The results indicate that IGC-AD1 clinically reduced agitation compared to placebo.
Read more here: IGC Pharma Announces Positive Interim Results for IGC-AD1 in Reducing Alzheimer’s Agitation (yahoo.com)
Agitation in Alzheimer’s Dementia refers to a range of behaviors that include various forms of verbal or physical aggression, as well as excessive motor activity, such as pacing, hiding and hoarding things, repetitive behaviors, among others1.

Agitation in Alzheimer’s Dementia refers to a range of behaviors that include various forms of verbal or physical aggression, as well as excessive motor activity, such as pacing, hiding and hoarding things, repetitive behaviors, among others1.

Agitation can be distressing for both patients and caregivers, leading to increased caregiver burden, reduced quality of life, and higher rates of institutionalization for patients2,3.
Agitation is associated with faster cognitive decline and accelerated progression of Alzheimer’s disease, making it a significant clinical concern4.

With over 50 million living with Alzheimer’s worldwide5 between 25 million and 40 million people suffer from agitation as a symptom of their disease. There is a significant unmet need for a safe medication to treat this medical condition.
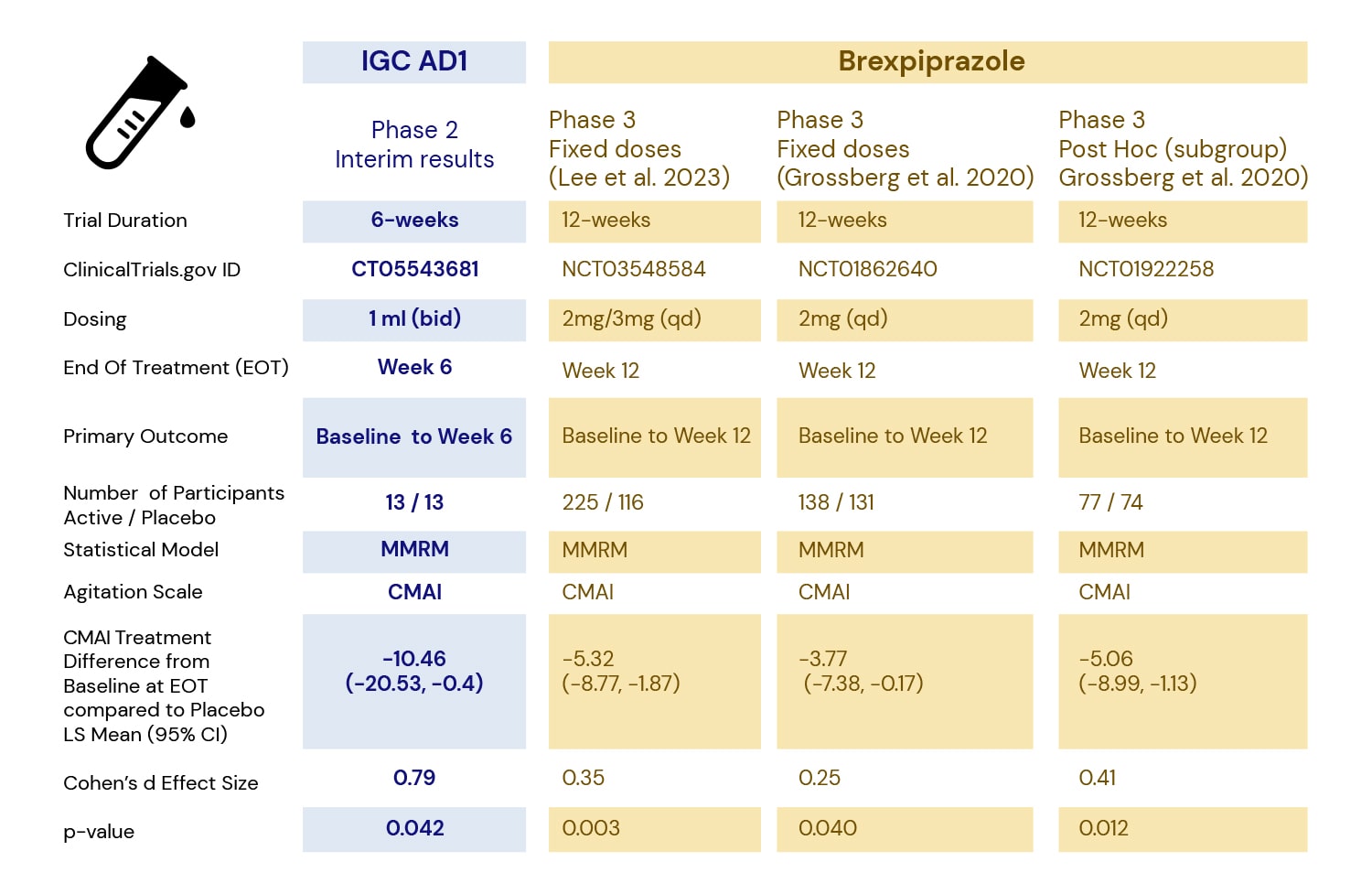
In 2023 the FDA approved Brexpiprazole, the only drug approved to treat agitation in Alzheimer’s. It is an atypical antipsychotic with a boxed warning. Our interim data set out in Table 1 shows that IGC-AD1 is safe and efficacious with a large effect size compared to placebo.
Table 1. Results of IGC-AD1 Interim Analysis and Illustrative Brexpiprazole* Phase 3 Results6.
Forward-Looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma’s expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma’s control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company’s failure or inability to commercialize one or more of the Company’s products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA’s general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma’s U.S. Securities and Exchange Commission (“SEC”) filings. IGC Pharma incorporates by reference the human trial disclosures and Risk Factors identified in its Annual Report on Form 10-K filed with the SEC on July 7, 2023, and Quarterly Report on Form 10-Q filed with the SEC on February 14, 2024, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur.
- Sano M et al. Agitation in cognitive disorders: Progress in the International Psychogeriatric Association consensus clinical and research definition. Int Psychogeriatr. 2024;36(4):238-250.
- Schein J et al. The Impact of Agitation in Dementia on Caregivers: A Real-World Survey. J Alzheimers Dis. 2022;88(2):663-677.
- Etters L et al. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract. 2008;20(8):423-428.
- Pless A et al. Understanding neuropsychiatric symptoms in Alzheimer’s disease: challenges and advances in diagnosis and treatment. Front Neurosci. 2023;17:1263771.
- Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
- The comparison is for illustrative purposes and is not intended to provide a direct comparison of effectiveness between IGC-AD1 and Brexpiprazole.
*Brexpiprazole, an atypical antipsychotic that comes with a black box warning, the only FDA-approved medication for treating agitation in Alzheimer’s dementia. Source:https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-agitation-symptoms-associated-dementia-due-alzheimers-disease